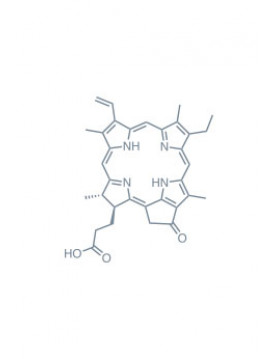
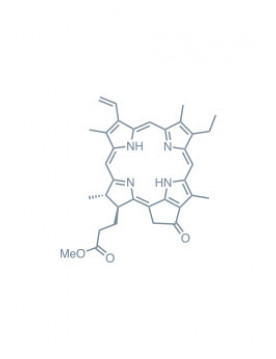
Chlorins
-
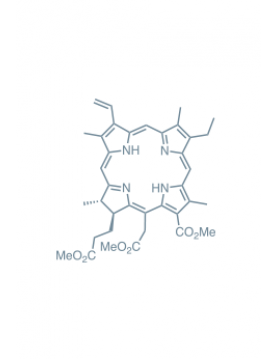
chlorin e6 trimethyl ester Learn More
-
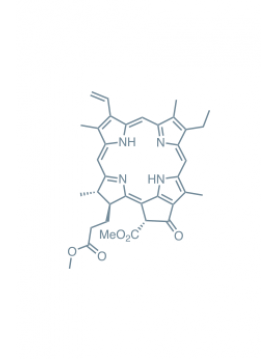
methyl pheophorbide-a Learn More
-
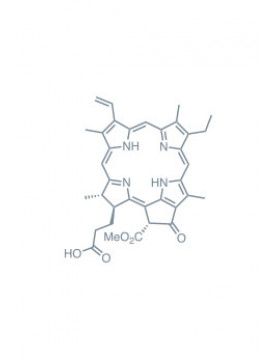
pheophorbide-a Learn More
-
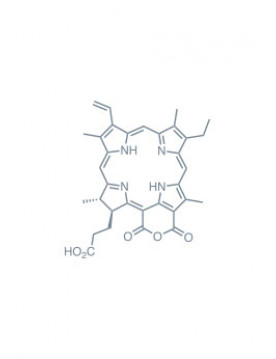
purpurin 18 Learn More
-
pyropheophorbide a Learn More
-
methyl pyropheophorbide-a Learn More
Chlorins
Introduction
Chlorins, natural derivatives of Chlorophyll a, have been garnering increasing scientific and pharmaceutical attention in recent years due to their considerable potential in diverse applications. This group of chemical compounds belongs to the family of porphyrins, vital molecules for life on earth due to their crucial role in biological processes such as photosynthesis and oxygen transport. In the pharmaceutical industry, chlorins have revealed significant applications in photodynamic therapy (PDT), an innovative and non-invasive approach for the treatment of various medical conditions, including cancer.
Chemical Properties and Structure
As previously mentioned, chlorins are derivatives of chlorophyll, a complex molecule that gives plants their distinct green color and is essential to photosynthesis. The structure of chlorins is characterized by a porphyrinic core comprising four pyrrole subunits linked by methine bridges, forming a macrocyclic structure. This gives chlorins a great ability to absorb light, particularly in the far-red region (650-800 nm), a key characteristic for PDT applications.
Chlorins are often modified to improve their chemical stability, increase their water solubility, and optimize their light absorption for maximum PDT efficacy. These modifications can include replacing the central magnesium with other metals, or demetallating the molecule to produce metal-free chlorins. Additional substitutions can also be introduced to adjust the physicochemical properties of the molecule, giving it great flexibility for various pharmaceutical applications.
Synthesis
The synthesis of chlorins from Chlorophyll A usually involves a series of degradation and modification steps. Various techniques, including chemical and enzymatic methods, can be used to produce chlorins with specific properties for various applications. The flexibility of these synthesis methods offers significant potential for the production of chlorins tailored to specific needs in the pharmaceutical industry.
Pharmaceutical Applications
Chlorins have found a particularly promising niche as photosensitizers in photodynamic therapy (PDT). In PDT, a photosensitizer is administered to a patient and selectively accumulates in diseased tissues. Then, the photosensitizer is activated by specific light, leading to the production of singlet oxygen, a highly reactive form of oxygen that can destroy diseased cells.
Chlorins are ideal candidates for PDT due to their strong absorption of light in the far-red region. This ability allows deeper penetration of light into tissues, enabling the treatment of deeper lesions. Moreover, chlorins tend to selectively accumulate in diseased tissues, which minimizes damage to surrounding healthy tissues during PDT.
Advantages and Challenges
Chlorins offer several significant advantages for PDT. Their strong absorption in the far-red means they can effectively treat deep lesions. Moreover, their tendency to selectively accumulate in diseased tissues allows for more targeted therapy with less damage to healthy tissues. However, there are also challenges to be addressed, such as the need to improve the stability of chlorins and develop methods to optimize their delivery to target sites.
Future Outlook
Chlorins continue to be intensively researched to improve their properties and develop new applications. For example, researchers are studying methods to make chlorins more stable and water-soluble, which would increase their applicability in PDT. In addition, work is underway to develop chlorins that can be activated by lights of different wavelengths, which would allow the treatment to be tailored to the specific needs of patients.
Conclusion
In conclusion, chlorins, derivatives of natural chlorophyll, have demonstrated considerable potential in the pharmaceutical industry, particularly in photodynamic therapy. Their ability to effectively absorb light, particularly in the far-red, and their tendency to selectively accumulate in diseased tissues, make them ideal candidates for targeted, non-invasive therapy. With ongoing research and the enhancement of synthesis and modification techniques, we can expect exciting new advancements in the use of chlorins in the years to come.